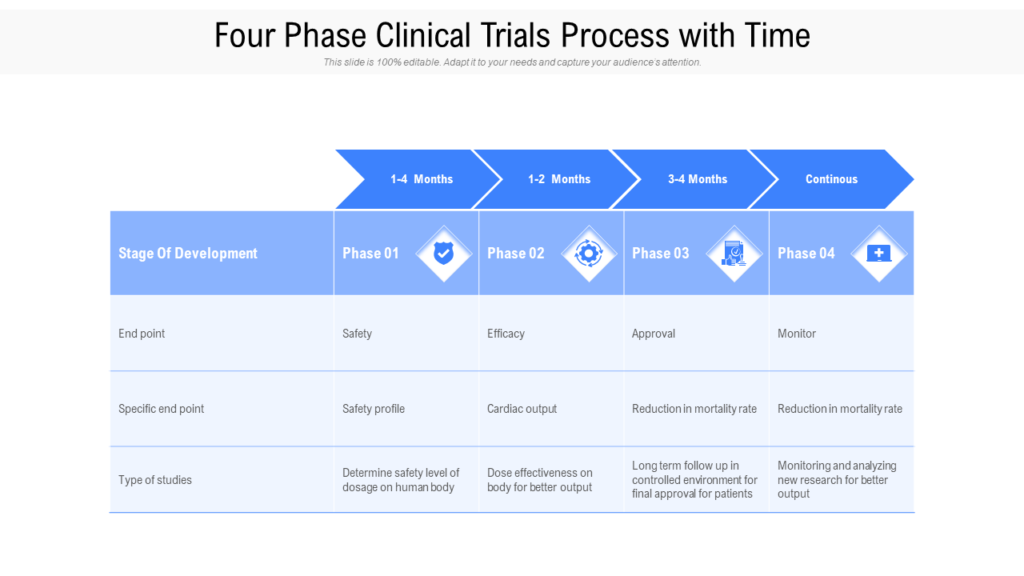
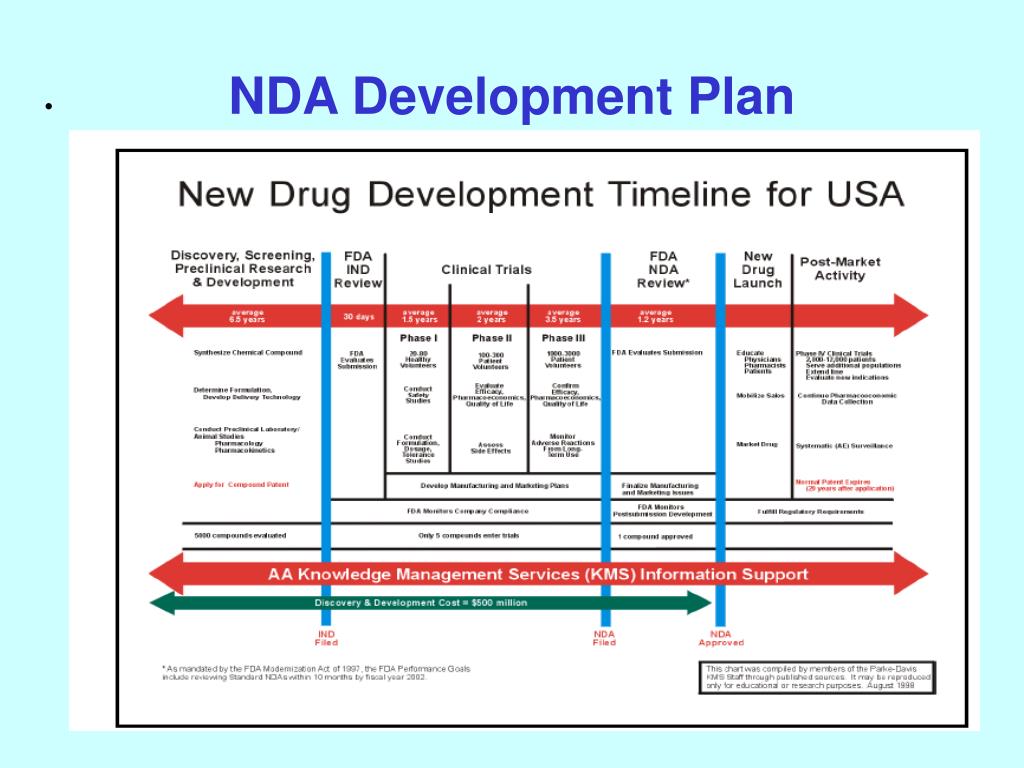
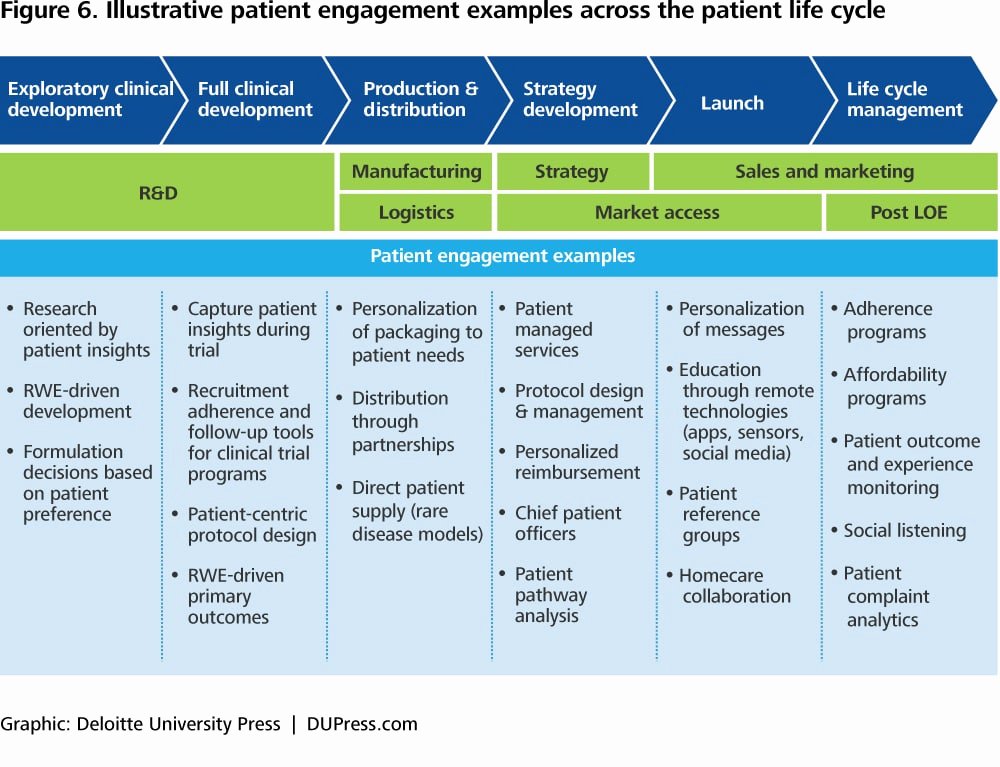
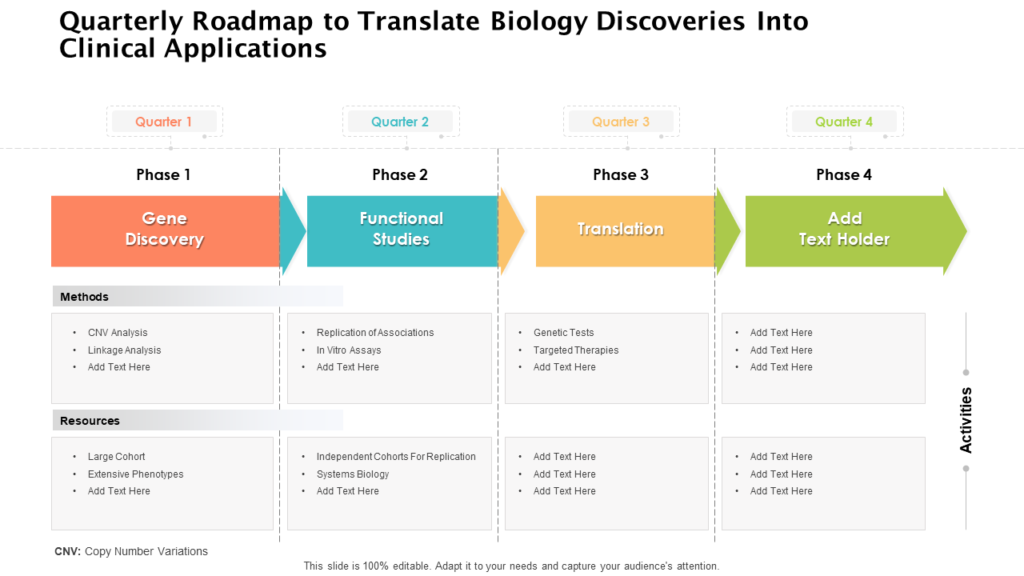
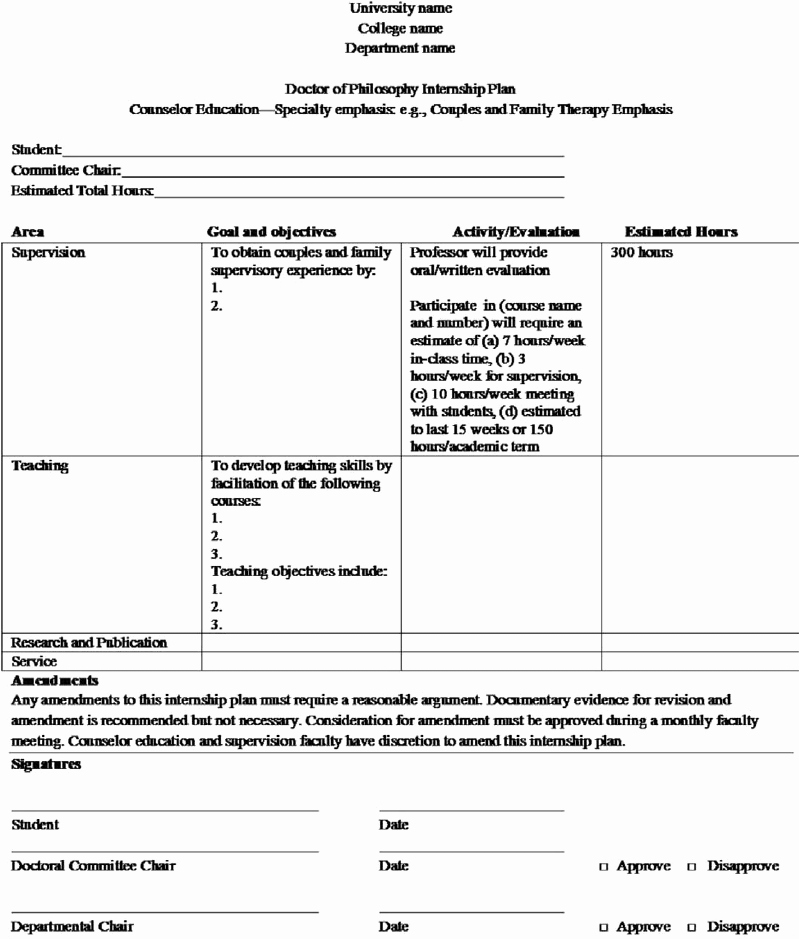
Clinical Development Plan Template Fda - Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The cdp should not to be confused. Ind content and format for phase 1 studies. I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Guidance for industry content and format of investigational new drug applications (inds). The second is the clinical development plan (cdp), which can be viewed as the map of how to get there.
Guidance for industry content and format of investigational new drug applications (inds). I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. The cdp should not to be confused. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. Ind content and format for phase 1 studies.
I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. The cdp should not to be confused. Guidance for industry content and format of investigational new drug applications (inds). The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Ind content and format for phase 1 studies. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism
Clinical Development Plan Template Fda prntbl.concejomunicipaldechinu
The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. Ind content and format for phase 1 studies. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and.
Clinical Development Plan Template Fda
The cdp should not to be confused. Guidance for industry content and format of investigational new drug applications (inds). I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism A target product profile.
Clinical Development Plan Template Fda
Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Guidance for industry content and format of investigational new drug applications (inds). I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. The second is the clinical development plan (cdp), which can be.
Clinical Development Plan Template
Ind content and format for phase 1 studies. A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism The.
30 Clinical Development Plan Template Hamiltonplastering
Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Guidance for industry content and format of investigational new drug applications (inds). The cdp should not to be confused. Ind content and format for phase 1 studies. The second is the clinical development plan (cdp), which can be viewed as the map of how to get there.
Clinical Development Plan Template Fda prntbl.concejomunicipaldechinu
A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Ind content and format for phase 1 studies. I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Guidance for industry content and format of investigational.
Clinical Development Plan Template Venngage
A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism The second is the clinical development plan (cdp), which can be viewed as the map of how to get there. Ind content and format for phase 1 studies. The.
Clinical Development Plan Template Fda
The cdp should not to be confused. I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism A target product profile (tpp) is a planning tool introduced by the fda to streamline the.
Clinical Development Plan Template Fda
Guidance for industry content and format of investigational new drug applications (inds). A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism Ind content and format for phase 1 studies. The second is the clinical development plan (cdp), which.
Clinical Development Plan Template
Clinical investigational plan synopsis • new onset atrial fibrillation • pulmonary embolism I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated. Guidance for industry content and format of investigational new drug applications (inds). The second is the clinical development plan (cdp), which can be.
The Cdp Should Not To Be Confused.
A target product profile (tpp) is a planning tool introduced by the fda to streamline the drug development process. Ind content and format for phase 1 studies. Guidance for industry content and format of investigational new drug applications (inds). The second is the clinical development plan (cdp), which can be viewed as the map of how to get there.
Clinical Investigational Plan Synopsis • New Onset Atrial Fibrillation • Pulmonary Embolism
I agree to comply with the protocol and ethical principles that have their origin in the declaration of helsinki and will follow the united stated.