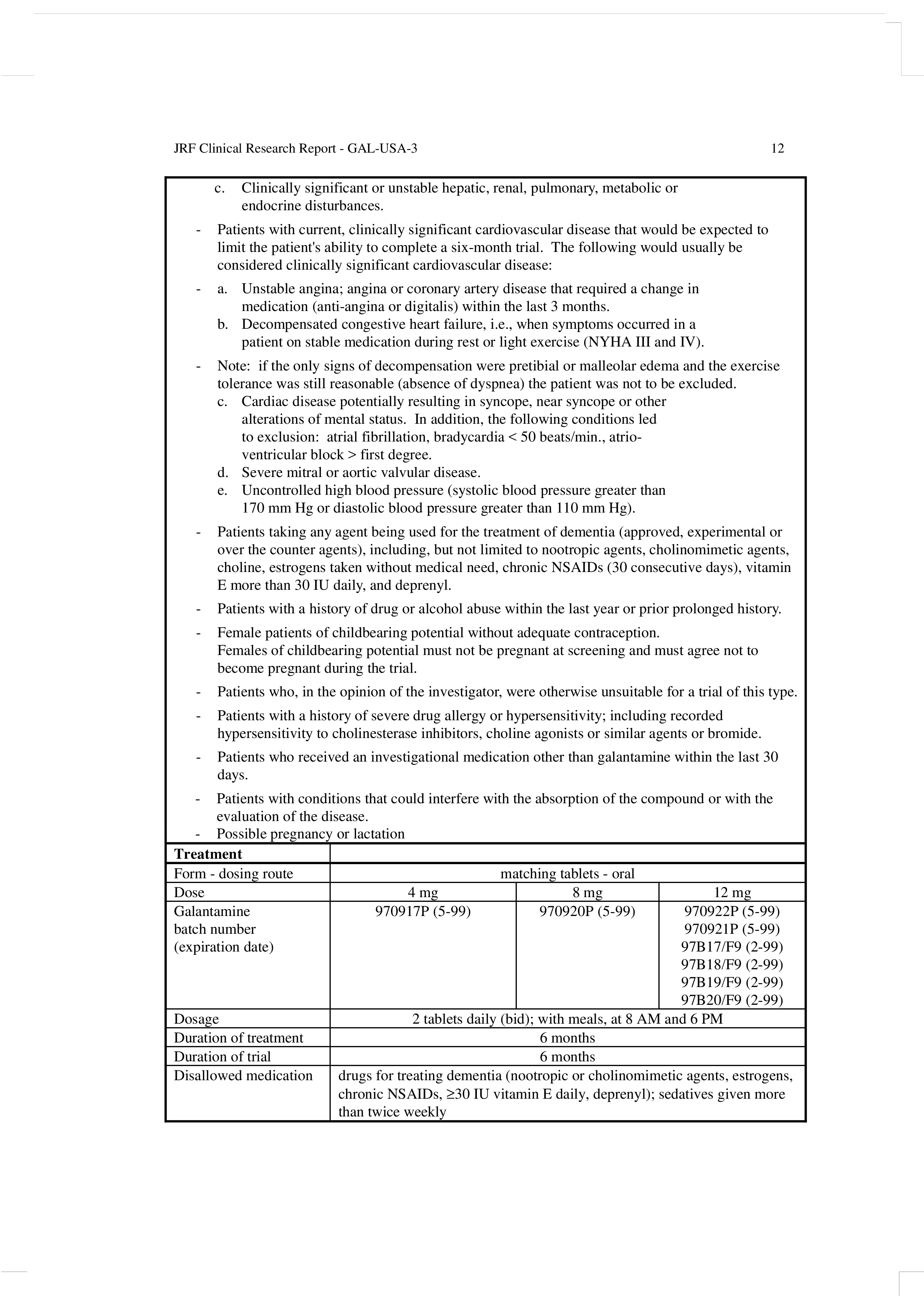
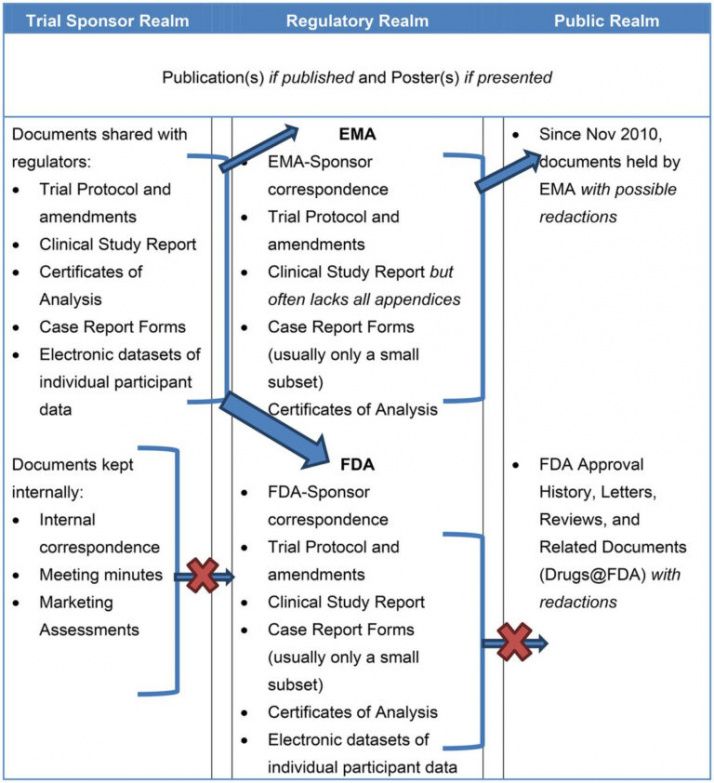
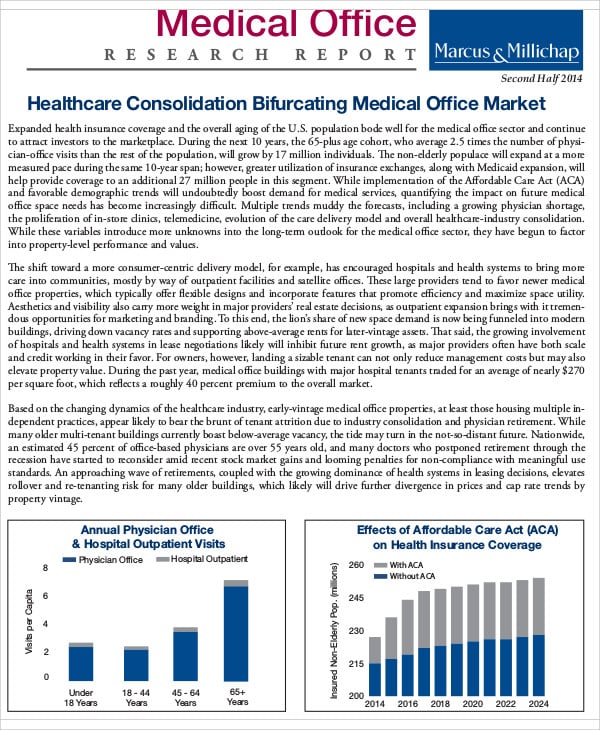
Clinical Study Report Template - This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. It covers topics such as.
This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. It covers topics such as. This document provides recommendations for the structure and content of clinical study reports submitted to the fda.
It covers topics such as. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This document provides recommendations for the structure and content of clinical study reports submitted to the fda.
Clinical Research Report Synopsis Templates at
This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. It covers topics such as. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. This document provides recommendations for the structure and content of clinical study reports submitted to.
Clinical Trial Report Template TEMPLATES EXAMPLE TEMPLATES EXAMPLE
This document provides recommendations for the structure and content of clinical study reports submitted to the fda. It covers topics such as. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs,.
Free Clinical Trial Templates Smartsheet
This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination.
Free Clinical Trial Templates Smartsheet
It covers topics such as. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document provides recommendations for the structure and content of clinical study reports.
Clinical Study Report Template PDF Sample Stableshvf
This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. It covers topics such as. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. This document provides recommendations for the structure and content of clinical study reports submitted to.
Clinical Study Report template
This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. It covers topics such as. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs,.
Clinical Study Report (CSR) Template Clinical Study Templates
Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. It covers topics such as. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2%.
a white paper with the words global health trust
Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document provides a note for guidance on the structure and content of clinical study reports for regulatory.
Clinical Trial Report Template (3) TEMPLATES EXAMPLE TEMPLATES
This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. It covers topics such as. This document provides recommendations for the structure and content of clinical study reports submitted to the fda. This document provides a note for guidance on the structure and content of clinical study reports for.
42+ Medical Report Samples Word, PDF, Illustrator
It covers topics such as. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. This document provides a note for guidance on the structure and content of clinical study reports for regulatory purposes. This document provides recommendations for the structure and content of clinical study reports submitted to.
This Document Provides A Note For Guidance On The Structure And Content Of Clinical Study Reports For Regulatory Purposes.
It covers topics such as. This is an abbreviated clinical study report (csr) of a phase iv study of brinzolamide 1%/brimonidine 0.2% fixed dose combination as. Find free and adaptable templates and tools for various aspects of clinical research, such as protocols, consent forms, logs, budgets, and. This document provides recommendations for the structure and content of clinical study reports submitted to the fda.